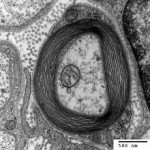
 All nerve cells are encased in sheath of myelin, which supports cells and acts as an electrical insulator allowing signals to be transmitted along the length of the axon (Hartline, 2008). Myelination of nerve cells usually starts during foetal development, and continues throughout childhood and adolescence (Paus et al., 1999). Loss of myelin can cause multiple sclerosis and Charcot Marie-Tooth disease (Bjartmar et al., 1999). Both diseases are characterised by a progressive loss of control of motor function, caused by loss of the protective myelin sheath and consequent nerve cell damage in the central and peripheral nervous systems respectively.
All nerve cells are encased in sheath of myelin, which supports cells and acts as an electrical insulator allowing signals to be transmitted along the length of the axon (Hartline, 2008). Myelination of nerve cells usually starts during foetal development, and continues throughout childhood and adolescence (Paus et al., 1999). Loss of myelin can cause multiple sclerosis and Charcot Marie-Tooth disease (Bjartmar et al., 1999). Both diseases are characterised by a progressive loss of control of motor function, caused by loss of the protective myelin sheath and consequent nerve cell damage in the central and peripheral nervous systems respectively.
Pemberton et al. recently reported that two breeds of dog, the Weimaraner and Chow Chow, are prone to a generalised tremor phenotype that appears around day 12-14 postpartum. In most cases, this tremor phenotype abated by 3-4 months of age, although some dogs retained a persistant fine tremor of hind limbs. The phenotype was more severe and persistant in Chows, often lasting to 4-5 months of age.
In both breeds, the tremor phenotype seems to be recessive. Pemberton et al. used genome wide association and haplotype analysis in three unrelated affected Weimaraner pedigrees to map the causative gene to a candidate region on chromosome 15. Sequencing of the 17 genes within this region revealed that the canine homologue of FLCN-interacting protein 2 (FNIP2) carried a truncating frameshift mutation in exon 9 that segregated with the tremor phenotype. A further 105 unrelated Weimaraners were sequenced for this mutation to determine its frequency in the breed. 9 out of 105 animals were heterozygous for this mutation, meaning that roughly 1 in every 136 Weimaraner dogs is likely to be affected by this tremor phenotype.
Histological analysis showed that in both Weimaraners and Chows, myelination throughout the central nervous system was reduced, and affected dogs had fewer mature oligodendrocytes – the cells that produce myelin in the central nervous system (Müller et al., 2013). Myelination increased over time, although did not reach the same levels as in wild type animals, which may explain why the tremor phenotype usually abates with time, but does not always disappear completely.
Direct sequencing of FNIP2 was not performed in Chows. However, the pups of a Weimaraner-Chow cross had a tremor phenotype and reduced central nervous system myelination, suggesting that FNIP2 is also the causative gene of the Chow tremor phenotype. Finally, the authors show that the murine homologue of FNIP2 is expressed in rat oliogdendrocytes, and its expression is regulated by Sox10, a known regulator of oligodendrocyte differentiation and function (Wegner and Stolt, 2005).
Together, these data suggest that FNIP2 may control myelination of the central nervous system in mammals. It has been suggested that protein and membrane trafficking is required for correct myelination (Krämer et al., 2001) and both FNIP2 and FLCN have been implicated in membrane trafficking (Nookala et al., 2012, Zhang et al., 2012), suggesting a mechanism through which FNIP2 might contribute to myelination.
At this stage it is unclear how or if this relates to FLCN function or BHD. As recessive mutations in FNIP2 are required to cause the tremor phenotype seen in these dogs, it is unlikely that dysmyelination is a concern for BHD patients. However, this study provides insights into the wider functions of FLCN and its interacting partner, FNIP2.
-
Bjartmar C, Yin X, & Trapp BD (1999). Axonal pathology in myelin disorders. Journal of neurocytology, 28 (4-5), 383-95 PMID: 10739578
-
Hartline DK (2008). What is myelin? Neuron glia biology, 4 (2), 153-63 PMID: 19737435
-
Krämer EM, Schardt A, & Nave KA (2001). Membrane traffic in myelinating oligodendrocytes. Microscopy research and technique, 52 (6), 656-71 PMID: 11276118
-
Müller C, Bauer NM, Schäfer I, & White R (2013). Making myelin basic protein -from mRNA transport to localized translation. Frontiers in cellular neuroscience, 7 PMID: 24098271
-
Nookala RK, Langemeyer L, Pacitto A, Ochoa-Montaño B, Donaldson JC, Blaszczyk BK, Chirgadze DY, Barr FA, Bazan JF, & Blundell TL (2012). Crystal structure of folliculin reveals a hidDENN function in genetically inherited renal cancer. Open biology, 2 (8) PMID: 22977732
-
Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, & Evans AC (1999). Structural maturation of neural pathways in children and adolescents: in vivo study. Science, 283 (5409), 1908-11 PMID: 10082463
-
Pemberton TJ, Choi S, Mayer JA, Li FY, Gokey N, Svaren J, Safra N, Bannasch DL, Sullivan K, Breuhaus B, Patel PI, & Duncan ID (2014). A mutation in the canine gene encoding folliculin-interacting protein 2 (FNIP2) associated with a unique disruption in spinal cord myelination. Glia, 62 (1), 39-51 PMID: 24272703
-
Wegner M, & Stolt CC (2005). From stem cells to neurons and glia: a Soxist’s view of neural development. Trends in neurosciences, 28 (11), 583-8 PMID: 16139372
-
Zhang D, Iyer LM, He F, & Aravind L (2012). Discovery of Novel DENN Proteins: Implications for the Evolution of Eukaryotic Intracellular Membrane Structures and Human Disease. Frontiers in genetics, 3 PMID: 23248642
www.bhdsyndrome.org – the primary online resource for anyone interested in BHD Syndrome.